Hot issues
Statistics in the agency’s Report on the State of Pharmaceutical Quality show that active pharmaceutical ingredient (API) sites that solely supply compounding pharmacies are disproportionately high risk.
The proposed updates aim to help address safety concerns created by pharmacies reselling compounded sterile drugs purchased from outsourcing facilities.
The Pennsylvania Attorney General filed charges against a pharmacy owner and the Ohio Board of Pharmacy revoked licenses to protect patients.
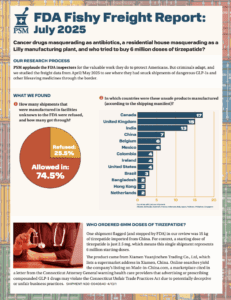
The newest Fishy Freight report continued to find suspect API shipped into the U.S. while a report from the United Nations’ Office of Drug Crime highlighted the dangers of contaminated and counterfeit medicines made with diethylene glycol and ethylene glycol.
What if your tirzepatide shipment came from a Brazilian beauty clinic? Or a vial of semaglutide was manufactured, supposedly, at a Costco in Toronto? In April and May 2025, dozens of shipments of semaglutide, tirzepatide, apixaban, and antibiotics entered the U.S. from facilities that aren’t in the FDA’s drug manufacturing database. These aren’t low-volume personal-use…
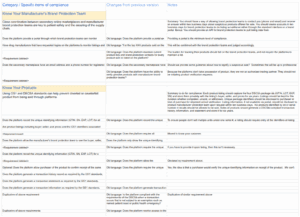
We’ve published the final document of best practices for online pharmacy-to-pharmacy marketplaces.
The Partnership for Safe Medicines (PSM) today announced its strong support for the reintroduction of the Cooper Davis Devin Norring Act, a bipartisan effort aimed at curbing the online sale of deadly counterfeit and illicit drugs.
Our podcast covers the latest in pharma crime and medicine safety.
Like your information on video? Subscribe to our YouTube playlist!

Who's investigating a company selling research-grade weight loss injections? Find out..

Updates on two federal prosecutions.

Click the images below to see more recent videos.